Fully enclosed light powder and heavy powder production system
The raw material drug production line realizes the closed transportation of materials, dust-free granulation and screening, dust-free granulation and closed metering and packaging. The production line operation process is fully enclosed, automated, energy-saving and highly efficient, in line with GMP specifications, and has passed the US FDA and EU COS dual system certification.
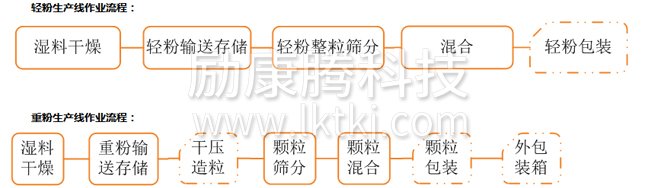
According to the production process, it can be divided into light powder production line and heavy powder production line.
Fully enclosed aseptic conveying, mixing, metering and filling system
The sterile powder injection production line realizes the closed turnover and mixing of sterile powder injection through vacuum conveying technology and airflow mixing technology, effectively avoiding the risk of material circulation exposure, and solving the problems of long material mixing time, low uniformity efficiency, and inconvenient cleaning. The air-flow mixing technology is used to replace mechanical operating parts to avoid the risk of foreign matter during the mixing process, with high product quality and high production safety. WIP cleaning, through steam damp heat sterilization, connection drying, saving cleaning time, low labor intensity, and high equipment use efficiency.